Theoretical Optimization of Design Parameters of Lithium-ion Battery Silicon-based Negative Electrodes
Heubner et al. considered the mixing ratio of silicon and graphite and the volume expansion of the material, and determined the optimal design criteria for the silicon-based porous electrode.
As a kind of anode material in the future, silicon has a theoretical capacity of about 4,200 mAh/g, which is more than 10 times higher than the 372 mAh/g of the graphite negative electrode. After its industrialization, silicon will greatly increase the capacity of the battery. However, the main problems existing in silicon materials are as follows: 1. When the charge and discharge are performed, the volumetric expansion reaches 300% to 400%; 2. The high irreversible capacity and low Coulomb efficiency lead to actual capacity loss and poor cycle life. After alloying with lithium, the volume of the silicon crystal changes significantly. This volume effect can easily cause the silicon negative electrode material to pulverize and peel off from the current collector. Moreover, the spalling caused by the volume effect of silicon can cause repeated destruction and reconstruction of the SEI, thereby increasing the consumption of lithium ions and eventually affecting the capacity of the battery. Currently, the above problems are being solved by means of nanometer silicon powder, silicon carbon coating, doping, and adhesive optimization.
From an engineering point of view, in order to increase the energy density of a battery, it is necessary to control the total mass of the electrode or battery. The quality of the electrode includes an active material, a liquid electrolyte filled in the electrode pores, a binder and a conductive additive, and a current collector. Therefore, the energy density of the electrode depends on the mass ratio of the active material to the non-active material used. Common technical approaches to increase the energy density of porous electrodes include increasing electrode thickness (active material/current collector ratio) and decreasing porosity (electrolyte/active material ratio). However, due to the limitations of lithium ion transport within the electrode, increased electrode thickness and reduced porosity all decrease the battery power density. In addition, the proportion of mixing with the graphite anode affects the capacity and average volumetric expansion of the composite electrode. Therefore, optimizing these design parameters is the key to the development of high-energy high-power lithium-ion batteries.
Heubner et al. considered the mixing ratio of silicon and graphite and the volume expansion of the material, and determined the optimal design criteria for the silicon-based porous electrode. They defined the "deformation threshold", due to the volume expansion of the silicon anode, the original porosity of the electrode will be filled to reduce the porosity, in order to avoid violent deformation and stress in the contact of the electrode particles during charging, and the sharp drop in porosity, There is a minimum in the initial porosity of the electrode. The electrode design must have a porosity greater than this value. In addition, a “rate current threshold†is also defined to ensure that the diffusion-limited current is not lower than the required rate current, thereby avoiding a significant reduction in capacity during fast charging. Then the influence of these design criteria on the performance parameters of the silicon-based negative electrode was analyzed, and the electrode design parameters were optimized to ensure the electrode energy density and power density by using the analytical relationship derived from the analysis.
1, porosity
The porosity ε0 of a lithium-ion battery electrode can be expressed by Equation (1):
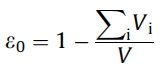 (1)
(1)
Vi is the volume of each solid component in the electrode, including silicon (Si), graphite (C), binder (B), and conductive agent (A). V is the overall volume of the electrode coating. Assuming SOC = 0 and SOC = 1, the volume change of each solid phase component is linear, and the expansion volume of each phase is n times the initial value, (the volume expansion of silicon, graphite, conductive agent, and binder are respectively For nSi = 3, nC = 0.1, nA = 0, nB = 0), considering this volume expansion, the electrode porosity ε(soc) in different SOC states is given by Equation (2):
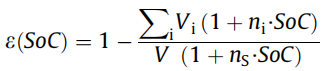
(2)
Assuming that the overall expansion of the battery is limited to ns times (eg, 10%) under the constraints of the battery outer case, the true density of each solid phase Ïi (the density of silicon, graphite, conductive agent, binder, and electrolyte, respectively) For ÏSi = 2336, ÏC = 2200, ÏA = 2200, ÏB = 1800, ÏCC = 8920, Ïel = 1500) and the mass percentage ωi, the equation (3) is obtained:
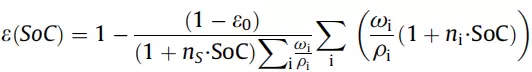
(3)
According to formula (3), for different initial porosity electrodes, the relationship between electrode porosity and SOC during lithiation is shown in Fig. 1a. Fig. 1b is a schematic diagram of the corresponding microstructure change (assuming that the electrode expansion is limited to ns = 10%). As the SOC increases, the porosity decreases significantly. When the initial porosity is in the range of 20 - 40% (the porosity of a typical commercial graphite electrode), the porosity of the silicon-based electrode will rapidly decrease to zero upon charging. Such a process can cause a great mechanical stress inside the electrode, causing silicon comminution, electrical contact failure, and the like, thereby reducing the capacity. In the case of medium initial porosity (50 - 70%), the decrease in porosity is less pronounced. However, if SOC = 1 is to be maintained, the electrode porosity does not fall to zero and the initial porosity needs to be more than 80%.
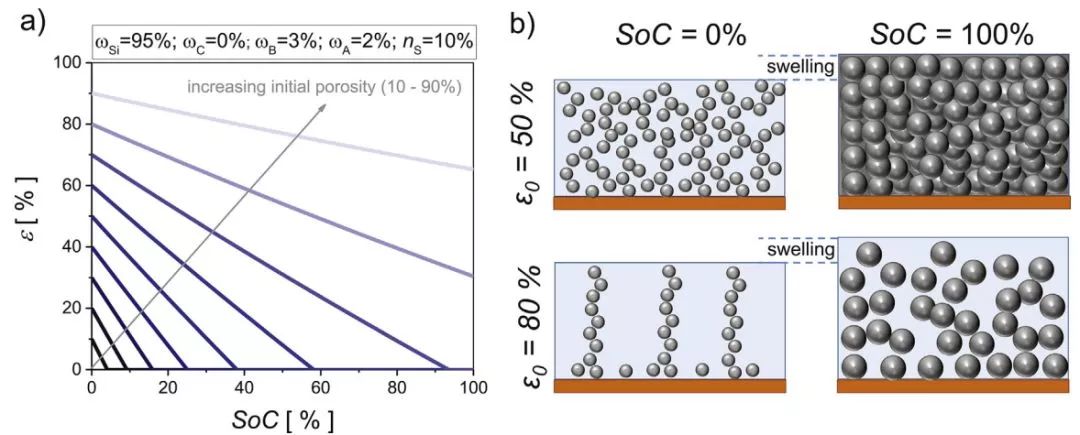
Fig. 1 (a) Evolution of porosity during lithiation at different initial porosities; (b) Evolution of electrode porosity at different initial porosities
Fig. 2a shows the relationship between porosity and initial porosity in the lithiation state of the electrode with different Si content. The increase of silicon content leads to a more dense electrode after lithiation. Pure graphite electrode, graphite volume expansion of 10%, if the electrode The volume change is limited to 10% and the lithiation does not change the porosity. Figure 2b shows the relationship between the porosity and the initial porosity in the lithiation state of three different silicon content electrodes under the limiting values ​​(0%, 10%, and 20%) of the whole electrode volume change for different electrodes. The smaller the value, the lower the porosity of the electrode after lithiation.
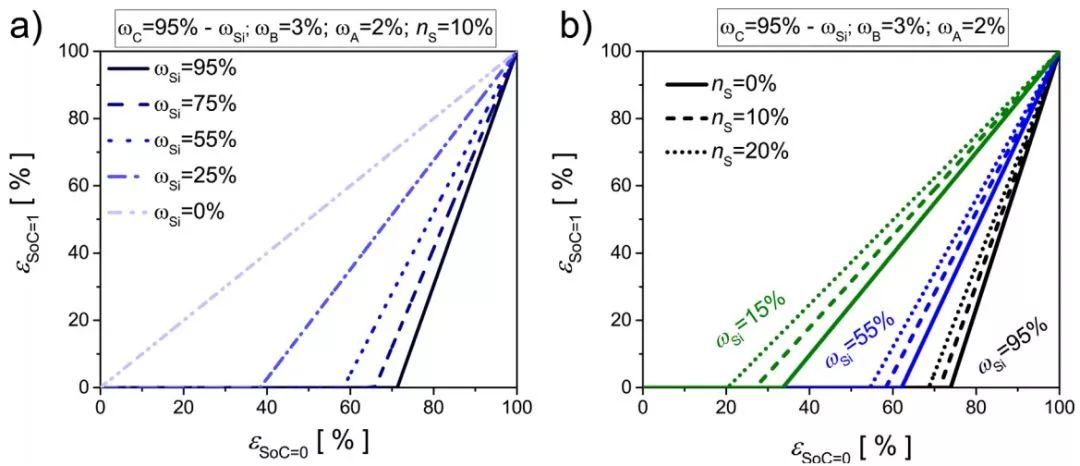
Fig. 2 (a) The SOC of the electrode with different silicon contents = 1 The relationship between the porosity and the initial porosity in the lithiated state; (b) The SOC of the electrode with different global volume change limit (ns) = 1 The pores in the lithiated state Relationship between rate and initial porosity
2. Distribution of electrolyte Li
In the lithiation reaction, lithium ions are inserted into the active material from the electrolyte, and the concentration of lithium in the electrolyte is reduced in the pores of the electrode. A concentration gradient is formed across the entire pole piece, causing lithium to diffuse to the negative pole. If the lithium concentration in the electrolyte drops to zero, the lithium insertion reaction stops. Therefore, the maximum current that can be reached, the so-called limiting diffusion current jlim, can be expressed as equation (4), and the effective diffusion coefficient is related to the porosity.

(4)
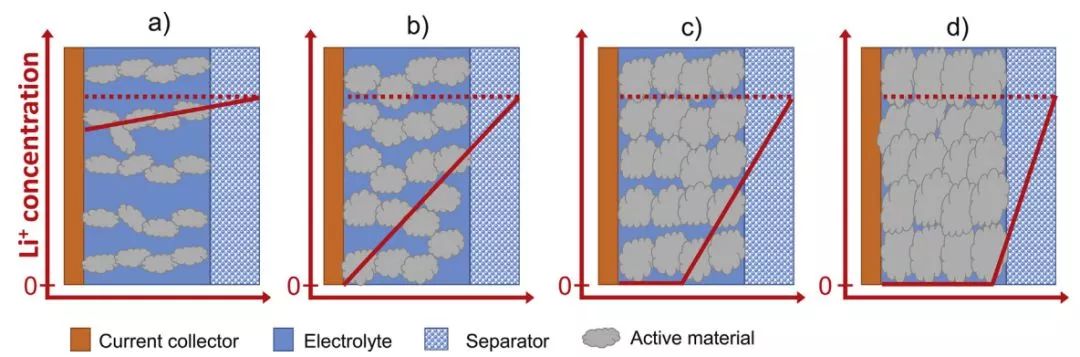
Fig. 3 Schematic diagram of the loss distribution of lithium concentration during constant current charging under different porosities
Fig. 3 is a schematic diagram of lithium concentration distribution in the electrolyte at constant current charge at different porosities. (a) Lithium transport in the electrode at large porosity is sufficient to make the lithium concentration in the electrolyte close to the initial value. (b) To reduce the porosity, the lithium ion concentration in the electrolyte gradually decreases to form a concentration gradient. (c) Further reducing the porosity, the lithium concentration inside the electrode is close to zero. (d) At very low porosity, the lithium concentration in the entire electrode rapidly decreases to zero.
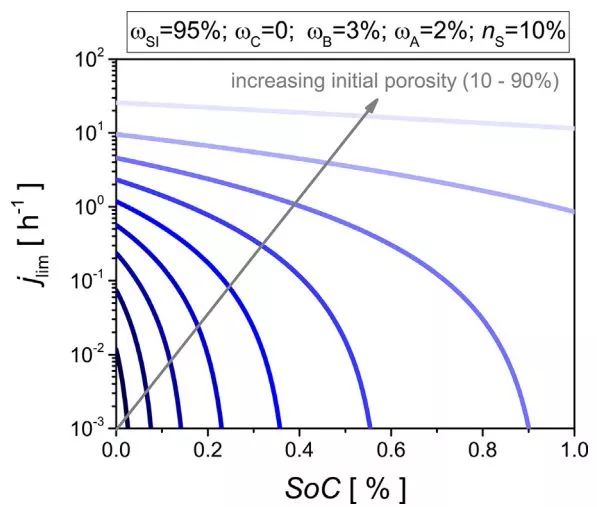
Fig. 4 Evolution of the diffusion-limited current rate during lithiation at different initial porosities
Figure 4 shows the evolution of the diffusion limit current rate during lithiation at different initial porosities. As the SOC increases, the rate performance decreases. For example, at an initial porosity of ε0 = 80%, the maximum diffusion-limited current of the electrode at SOC = 0 is 9.6 C, and SOC = 1 is about 0.85C.
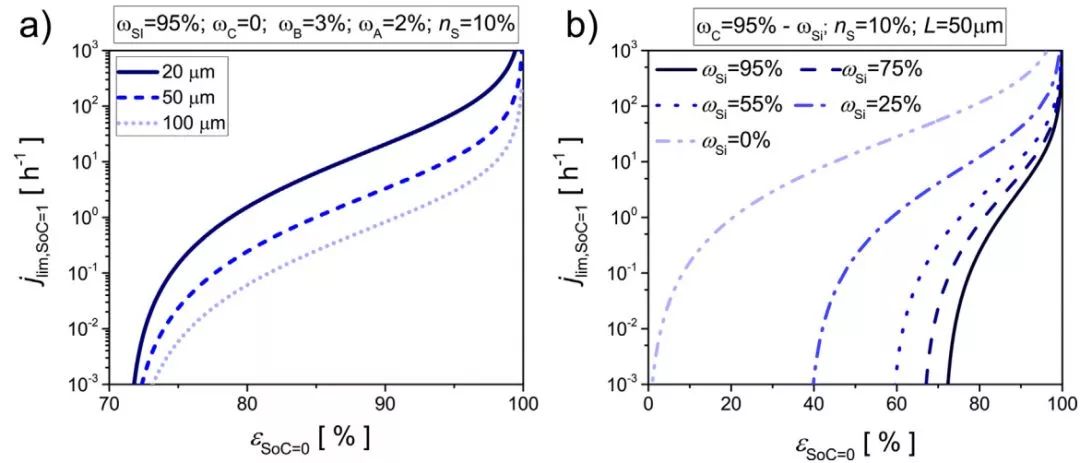
Fig. 5 (a) Diffusion limiting magnification and initial porosity in different electrode thicknesses and (b) different silicon contents
FIG. 5 shows the relationship between the diffusion limiting magnification and the initial porosity at different electrode thicknesses and different silicon contents. As the initial porosity increases, the rate performance increases. At a certain initial porosity, the diffusion-limited current decreases as the thickness of the electrode increases. Electrodes that are particularly thick or have extremely small pores are generally limited by diffusion, and the maximum charge-discharge rate decreases drastically when SOC=1. In addition, increasing the graphite content in the composite can significantly increase the rate performance of the electrode.
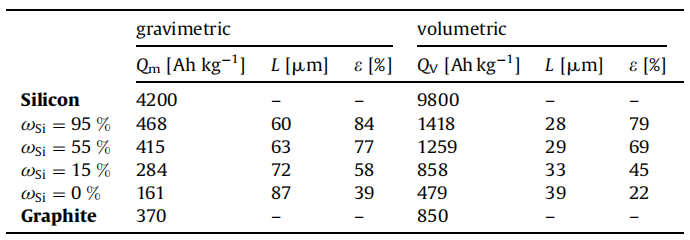
Conclusion: Considering the huge volume expansion effect of the silicon negative electrode, the porosity of the electrode will be reduced during the expansion process, and the stress between the particles will increase, resulting in powdering. Therefore, for the silicon carbon anode, the cell pole piece design should have a larger porosity than the graphite anode. Calculated theoretically, taking into account the mass and volumetric capacity, the different silicon content corresponds to the maximum specific capacity. In this case, the optimized electrode thickness and porosity are shown in Table 1.
Table 1 The maximum specific capacity and the corresponding optimal electrode thickness and porosity for silicon-carbon anodes with different silicon contents
Shenzhen Huiyunhai Tech.Co., Ltd. , https://www.cablesleevefactory.com